Yes. The term "self-emulsifying" refers to the ability to form stable microemulsions (definition of microemulsion: a homogeneous dispersion of oily substances in water, with a particle diameter of less than 0.1 micron). Nicomenthyl is an amphiphilic substance, which means it is both hydrophilic and lipophilic, as its molecule contains a pyridinic aromatic ring represented by nicotinic acid ("hydrophilic head") and a nonpolar portion represented by menthol ("hydrophobic tail").
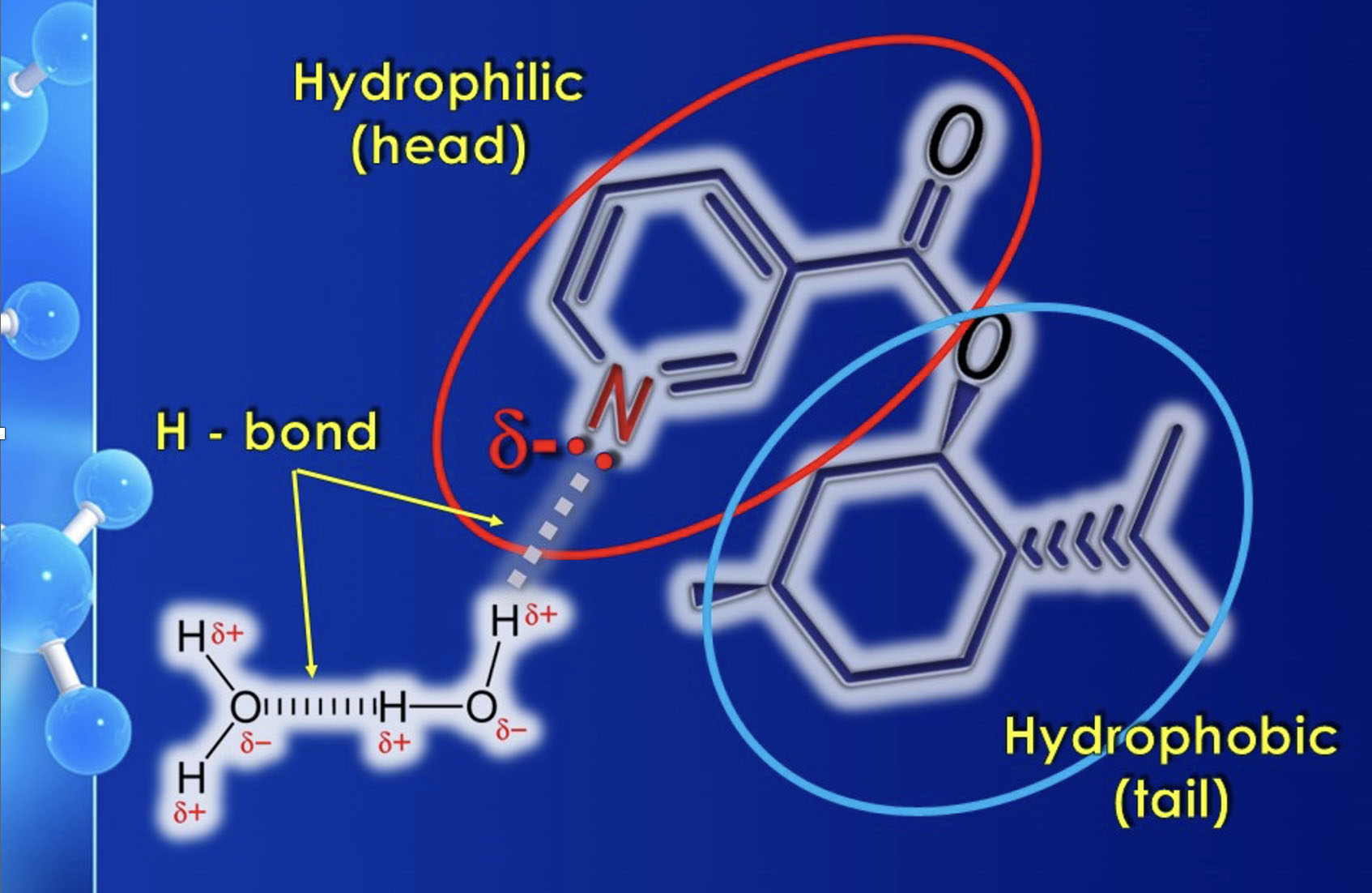
Through the hydrophilic head (nicotinic acid), interactions with water molecules can occur due to the formation of either hydrogen bonds or dipole-dipole interactions. This is what makes Nicomenthyl water-dispersible and, therefore, capable of forming stable microemulsions. However, this does not mean that Nicomenthyl is an "emulsifier" or a "surfactant" by definition. It simply means that it can be easily dispersed in an aqueous medium. (the "Ouzo effect": https://youtu.be/t5jbxh0C0UU?si=uEVW5BFUxXsL7o0B) with the aid of any co-emulsifiers or solubilizers.
